Similarities and Differences Between Hydrolysis and Dehydration Synthesis
Biology QA Library Compare and contrast hydrolysis and dehydration synthesis reactions. Dehydration synthesis is like the opposite of hydrolysis.

Dehydration Synthesis And Hydrolysis De Away From Hydration Relating To Water Synthesis To Put Together Hydro Relating To Water Lysis Ppt Download
In hydrolysis water is added.
. There are two processes that play vital roles in biosynthesis. Both dehydration synthesis and hydrolysis deal with water but in opposite waysDehydration synthesis rips H2O away from two molecules so it can combine the two molecules. 6 rows Hydrolysis is the decomposition reaction whereas dehydration synthesis is a combination.
Similarities between hydrolysis and dehydration synthesis next page Good discursive essay topics higher Help with college essay professional american writers brilliant essay is offering your with online college with writing essay questions help all research work writing skills and project management essay writing tips. Difference Between Hydrolysis and Dehydration Synthesis Hydrolysis is a process where a water molecule is added to a system but dehydration synthesis is a process where a. Essay introduction however recent.
Usually it is termed dehydration condensation. Hydrolysis and Dehydration Synthesis both deal with water and other molecules but in very different ways. Dehydration is the condition of having less amount of water than the normal level.
What is the difference between Hydrolysis and Dehydration. Hydrolysis reactions largely occur inside the cell Dehydration reactions remove water from the cell. For example if we wanted to compare physical changes with chemical changes we could compare and contrast them like this.
Explain in terms of dehydration synthesis and hydrolysis reactions the relationships between starch in an ingested potato liver glycogen and blood glucose. What are the similarities and differences between a fat and an oil. These are Hydrolysis and Dehydration Synthesis.
Biological macromolecules are organic because they contain carbon. Dehydration reactions typically require an investment of energy for new bond formation while hydrolysis reactions typically release energy by breaking bonds. See answer 1 Best Answer.
In this case by removing a molecule of water two small molecules can connect to form a larger. Dehydration synthesis bonds molecules together by removing water. Dehydration synthesis and hydrolysis similarities.
Dehydration synthesis leads to formation of monomers polymers water and polymers. Compare and contrast hydrolysis and dehydration synthesis reactions. Compare and contrast dehydration synthesis and hydrolysis.
The difference between hydrolysis and dehydration is that hydrolysis is the breakdown of larger molecules into smaller ones lysis whereas dehydration is the removal of water with the help of a dehydrating agent to yield a new product. The former results in the combination of monomers to form polymers and the latter takes place when polymers are split. Describe the similarities and differences between glycogen and starch.
A hydrolysisreaction is when a. Dehydration and hydrolysis reactions are similar for all macromolecules but each monomer and polymer reaction is specific to its class. School uniforms this work has been published in the teen ink monthly print magazine until college i wore uniforms all my life in school they can be.
Dehydration Synthesis and Hydrolysis. Dehydration synthesis and hydrolysis refer to two opposite organic reactions. Comment on the physiological and clinical significance of the degree of saturation of fatty acid chains.
Record this using the template for a comparative contrastive map see the overall map idea below. Dehydration affects hydrolysis reactions because for the hydrolysis reactions to take place there should be water. Hydrolysis breaks down molecules by reacting with water.
First week only 499. In dehydration synthesis reactionsa water molecule is formed as a result of generating a covalent bond between two monomeric components in a larger polymerIn hydrolysis reactionsa water molecule is consumed as a result of breaking the covalent bond holding together two components of a polymer. Dehydration synthesis is when two molecules are chained togetherand a water molecule is ejected from the coupling.
Essentially the concept involves adding of water molecule to another molecule to break the bond or bridge and form a new chemical compound. Both have a reverse reaction in. Dehydration reactions and hydrolysis reactions both assemble polymers from monomers Dehydration reactions largely occur outside of the cell.
The Difference between Hydrolysis and Dehydration Synthesis is that dehydration synthesis results in the formation of bigger molecules by joining smaller molecules while hydrolysis is the breakdown of large molecules into smaller ones. Look for similarities and differences between the pair of changes. They are similar because they both deal with water.
What role do electrons play in dehydration synthesis and hydrolysis. Hydrolysis on the other hand is simple the reverse of dehydration synthesis mechanism. Hydrolysis separates molecules into parts mostly and dehydration synthesis condenses molecules into a larger.
Both of these reactions involve water. Compare and contrast hydrolysis and dehydration synthesis reactions. Start your trial now.
Hydrolysis is a reaction where a chemical bond is broken using a water molecule. What summarizes the similarities or differences between hydrolysis reactions and dehydration reactions. What is the similarities between dehydration synthesis and hydrolysis.
Before you thank dealing with the criteria for essay good-gets-and-obsessive-compulsive-disorder 2008. The difference between dehydration synthesis and hydrolysis is that in one bonds are being formed while in the other bonds are being destroyed.

Dehydration Synthesis And Hydrolysis Youtube
What Is The Difference Between Dehydration Synthesis And Hydrolysis Quora

Dehydration Synthesis Definition Examples And Equations

Dehydration Synthesis And Hydrolysis De Away From Hydration Relating To Water Synthesis To Put Together Hydro Relating To Water Lysis Ppt Download

Difference Between Hydrolysis And Dehydration Synthesis Explained

Dehydration Synthesis Definition Examples And Equations

Difference Between Hydrolysis And Dehydration Synthesis Differbetween
Difference Between Hydrolysis And Dehydration Synthesis Difference Between

Carbohydrates L3 Biology Make The Above Structures Hydroxyl Group On Carbon Perform Dehydration Synthesis Ppt Download
Synthesis Of Biological Macromolecules Boundless Biology
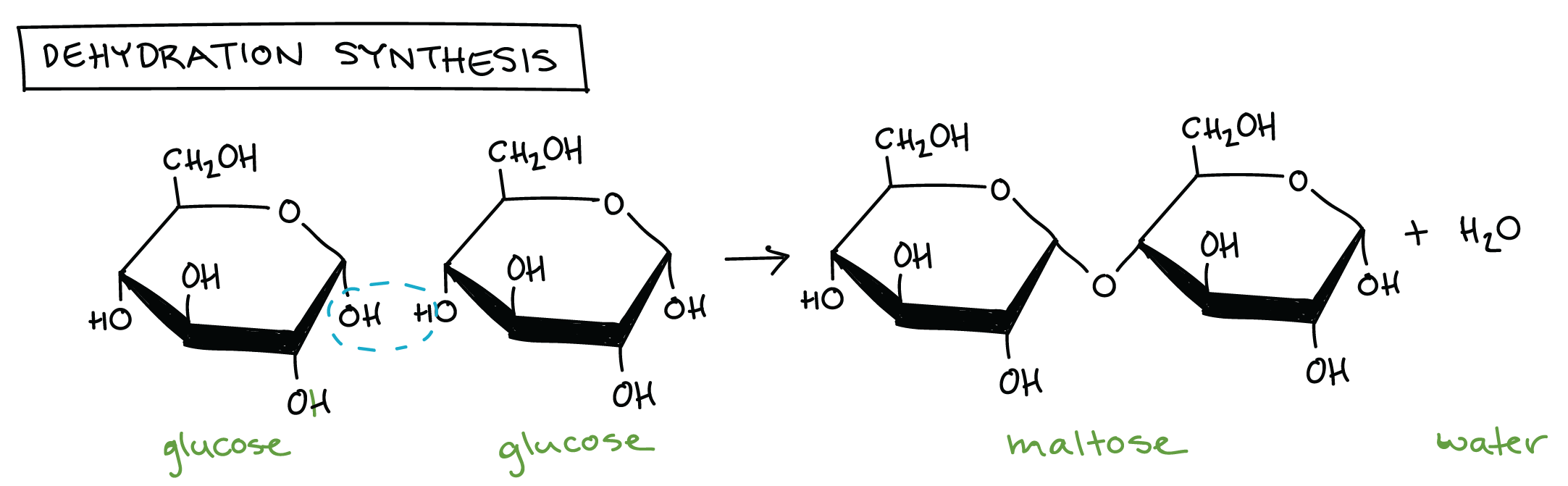
Introduction To Macromolecules Article Khan Academy
Difference Between Dehydration Synthesis And Hydrolysis Definition Mechanism Examples
Difference Between Hydrolysis And Dehydration Synthesis Difference Between

File 213 Dehydration Synthesis And Hydrolysis 01 Jpg Wikiversity
Difference Between Dehydration Synthesis And Hydrolysis Definition Mechanism Examples
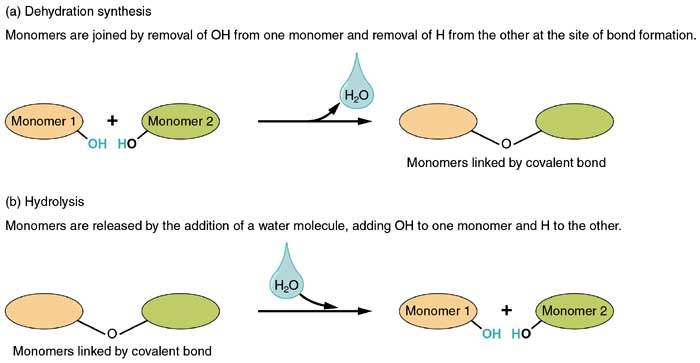
Dehydration Synthesis And Hydrolysis Types Reactions Roles

Hydrolysis And Dehydration Synthesis Reactions Youtube

File 213 Dehydration Synthesis And Hydrolysis 01 Jpg Wikiversity
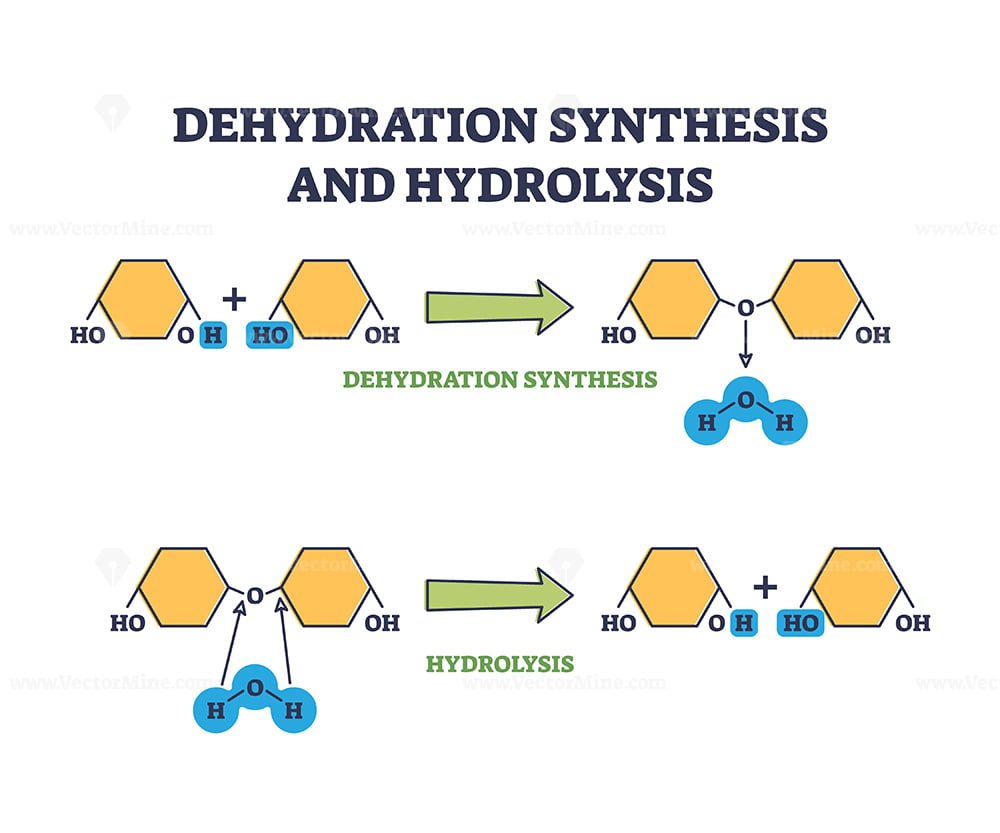
Dehydration Synthesis And Hydrolysis Chemical Process Stages Outline Diagram Vectormine
Comments
Post a Comment